The world wants the fuel cell to work. In the past few months, the Department of Energy has thrown a few extra million dollars into fuel cell research, General Motors and Honda teamed up to develop a fuel cell to work in their cars within the decade, and the Swiss have introduced a fuel cell-powered coffee trolley on their trains.
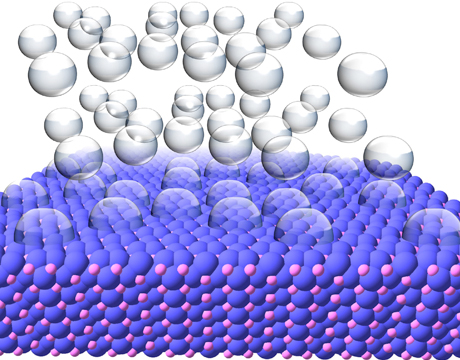 |
| Hydrogen gas bubbling off of the surface of a nickel phosphide crystal. Image: Eric Popczun, Penn State University |
Of course, fuel cells already do work.The trick to making the green technology dominate as an energy source is, as always, finding a way to bring the cost down. One big impediment to making fuel cells affordable is the price of hydrogen. It is everywhere, but it rarely shows up on the planet in an elemental state. To harvest hydrogen, a platinum catalyst is used to separate it from the oxygen in H2O. At the moment, the rare metal sells for about $1,500 an ounce.
Now Raymond Schaak, a professor of chemistry at Penn State University has found an alternative catalyst material: nickel phosphide nanoparticles. Though not quite as common as dirt, the building blocks for the material are a lot less rare than platinum.
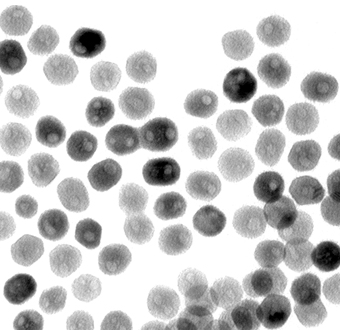 |
A transmission-electron microscope image of a collection of quasi-spherical nickel phosphide nanoparticles. Image: Eric Popczun, Penn State UniversitySpherical Splitting
The particles are effective at splitting water, most likely, because they are spherical, with many flat facets. “In general, nanoparticles have very high surface area compared to their bulk counterparts, and this gives overall higher activity on a per-atom basis simply because a larger fraction of the atoms in the overall sample are located at the surface, where catalytic reactions happen,” explains Schaak. “However, we believe that the flat sides—the “facets”—help increase the activity further, especially since one of the exposed facets is the exact surface predicted by theory to be the most active.” Schaak guesses that a non-nano powder would still be effective, just less so, since the surfaces area would be smaller.
When charged, the nanoparticle is comparable “to the best known nonplatinum catalysts under similar working conditions,” he says.
Kinks in the Catalyst
A catalyst is supposed to passively do its job without any worries of being used up. In practice, that isn’t always the case. Currently the catalyst does suffer some loss in activity after a long period of use. “This is due either to degradation of the catalyst or simply the removal of the particles from the surface of our electrode used for testing our materials,” says Schaak. It’s just one of many items that need to be ironed out.
Another is the cost of production. Right now, producing the particle as Schaak does in his lab would be prohibitively expensive. But once Schaak and his colleagues and students in the Schaak group, determine which aspects of the material make it work—the shape, the surface area, or some other piece of its chemistry—they could focus on creating variations with a stronger emphasis on those features. The result could be an even more effective material with a much cheaper production processes.
Such variations may lead to a similarly behaving but fundamentally different material. “We believe that this is a first step toward discovering many other new catalytic materials,” says Schaak. “So, one challenge is to fully explore related materials in order to identify those that are the best, which may or may not be this nickel phosphide. There is also much optimization that would be required, including to increase the performance and stability of the catalyst, scale up its production, and fully understand mechanistically how the hydrogen evolution reaction is catalyzed by this complex material.”
Perhaps, after all that, hydrogen will flow for all, and a cheap, efficient fuel cell will hit the streets (to say nothing of a superior solar cell). But, as Schaak puts it, “This discovery is pretty fundamental and scientific in nature at this point.”
|


.jpg)

0 comments:
Post a Comment